NDTV, India’s first privately owned TV news channel, quoted Adar Poonawalla, the chief of Pune-based Serum Institute of India – world’s largest vaccine maker, as saying, “We hope to start producing this [coronavirus] vaccine by May-end so that by the time the trials get over in September, we could have a product that we can give to the Indian people and the world and not have to wait another six months after that to manufacture the vaccine.” He was referring this week to the trial under-way at Oxford University’s Jenner Institute, which the New York Times recently reported as “in race for a coronavirus vaccine, an Oxford Group leaps ahead”.
– TIN Bureau
COVID-19 is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 was first detected in the city of Wuhan, China, in December 2019, after a cluster of patients with pneumonia of unknown cause were reported to the World Health Organization (WHO). The outbreak was declared a public health emergency of international concern on 30th January 2020, and the disease caused by SARS-CoV-2 was officially named COVID-19 on 11th February 2020. After assessing the outbreak and following transmission of the virus in many other countries worldwide, on 11th March 2020 the WHO declared COVID-19 a pandemic. This means that the disease has spread worldwide, and it is the first time that a coronavirus has led to a pandemic.
Scientists around the world are working hard to develop a vaccine to prevent COVID-19, but there is a lot to be done. A team in Oxford led by Prof. Sarah Gilbert, Prof. Andrew Pollard, Prof. Teresa Lambe, Dr Sandy Douglas and Prof. Adrian Hill started work designing a vaccine on Saturday 10th January 2020. The current status is that they have identified a vaccine candidate and are working towards the first clinical testing phase.
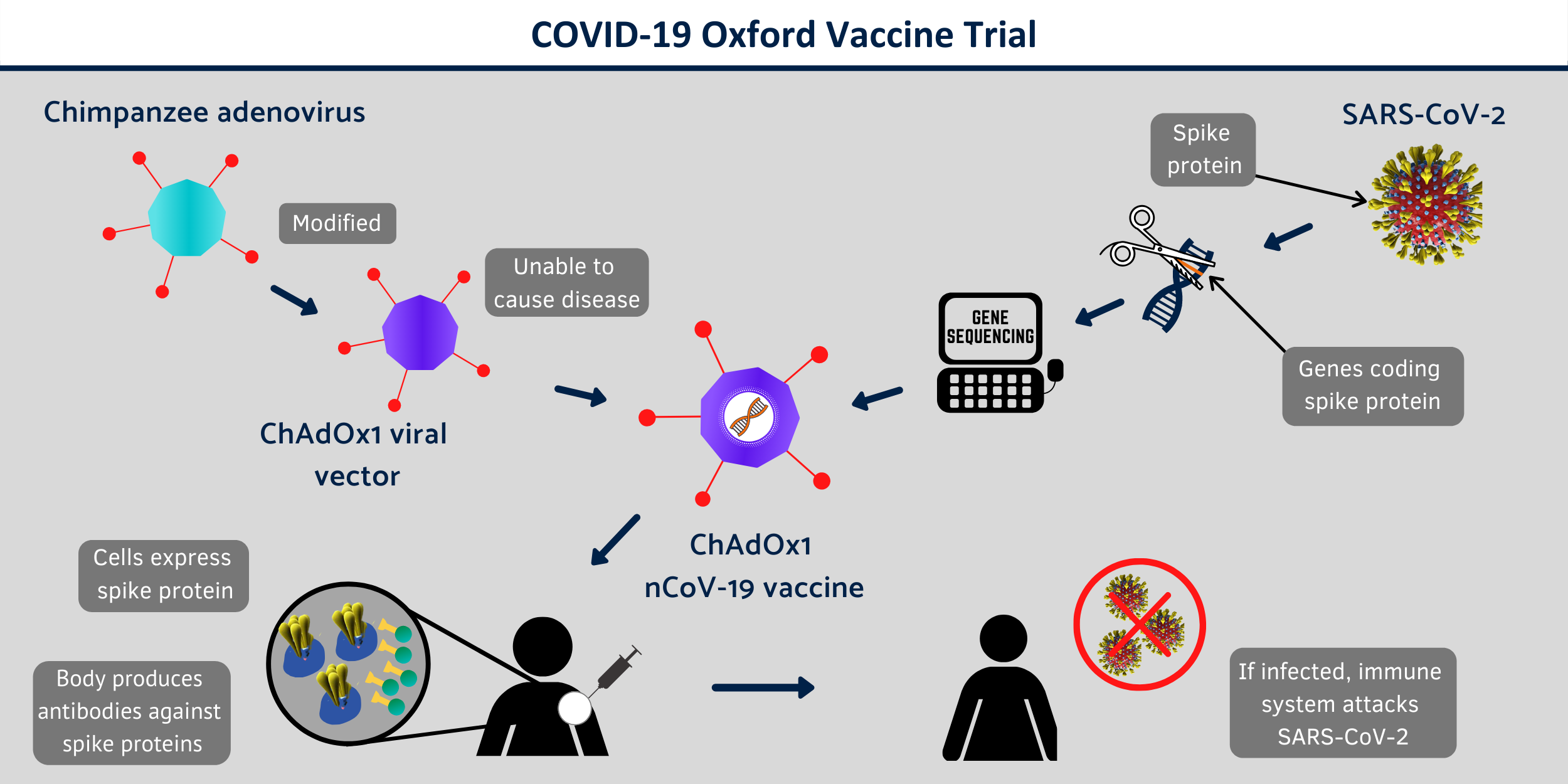
A chimpanzee adenovirus vaccine vector (ChAdOx1), developed at Oxford’s Jenner Institute, was chosen as the most suitable vaccine technology for a SARS-CoV-2 vaccine as it can generate a strong immune response from one dose and it is not a replicating virus, so it cannot cause an ongoing infection in the vaccinated individual. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Chimpanzee adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects, from 1 week to 90 years of age, in vaccines targeting over 10 different diseases.
Coronaviruses have club-shaped spikes on their outer coats. Immune responses from other coronavirus studies suggest that these are a good target for a vaccine. The Oxford vaccine contains the genetic sequence of this surface spike protein inside the ChAdOx1 construct. After vaccination, the surface spike protein of the coronavirus is produced, which primes the immune system to attack the coronavirus if it later infects the body. Prof. Gilbert and team have previously developed a vaccine for another human coronavirus disease, which is Middle East Respiratory Syndrome (MERS), and this has shown promise in early clinical trials.
At the same time as preparing for and conducting the first clinical trial, production of the vaccine is being scaled up ready for larger trials, and potentially, future deployment.
Researchers at Oxford University are working with great care, and due haste, in developing a new vaccine for coronavirus. If proven effective, a safe coronavirus vaccine could provide an exit strategy for the pandemic and save lives.
The ChAdOx1 nCoV-19 vaccine timeline
We must remain cautious when mapping out a timeline for the trial. The plans for the clinical trial are as follows:
Phase I – Vaccinate 510 volunteers aged between 18-55, half with the new COVID-19 vaccine and half with a control vaccine.
Phase II – Extend the maximum age of trial participants to 55-70 years, then over 70.
Phase III – Vaccinate 5000 volunteers aged over 18 years, half to receive the COVID-19 vaccine. Clear efficacy endpoints will be used to assess the effectiveness of the vaccine, and volunteers from phase I and II will be included in the follow-up.
The best-case scenario is that by the autumn of 2020 we could have an efficacy result from the phase III trial to show that the vaccine protects against the virus, alongside the ability to manufacture large amounts of the vaccine, but these best-case timeframes are highly ambitious and subject to change.
(All the above info & picture is courtesy www.covid19vaccinetrial.co.uk)